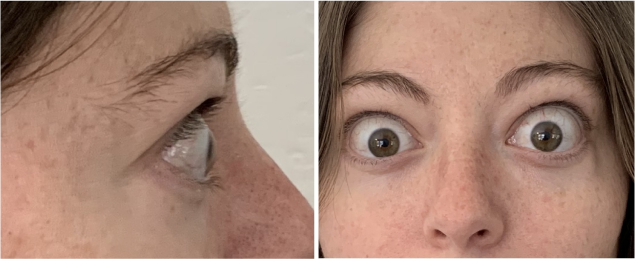
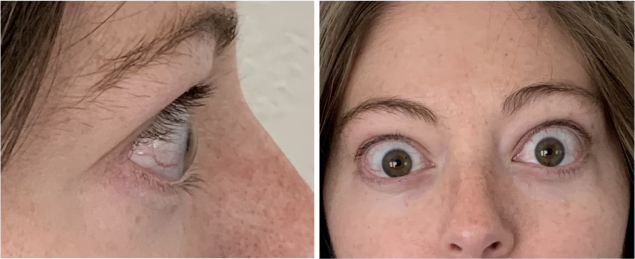
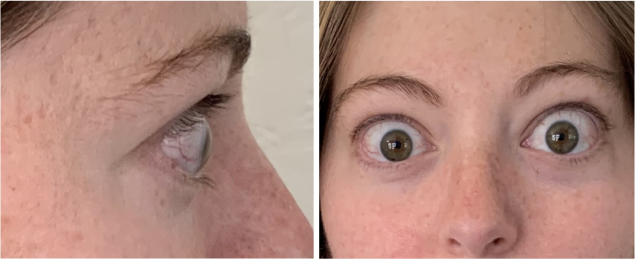
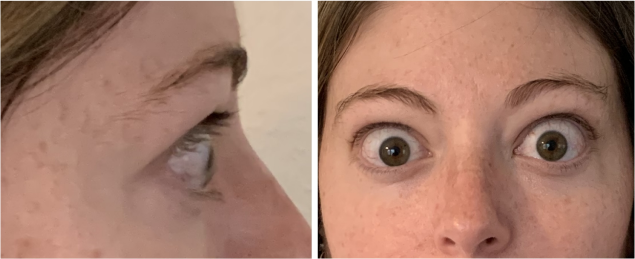
Now approved in Saudi Arabia
Start treatment for Thyroid Eye Disease (TED) earlier.
With TEPEZZA®
Start treatment for Thyroid Eye Disease (TED) earlier. With TEPEZZA®
TEPEZZA is the first and only approved treatment for TED1,2
The first and only human monoclonal IGF-1R inhibitor designed to block the autoimmune reaction at its source1,3-7
Generally well tolerated1,9,10
Significantly reduced the devastating clinical, psychological, and social consequences of the disease1,8-11
Treatment response with only 8 infusions1,9,10
About Thyroid Eye Disease (TED)
TEPEZZA is indicated for the treatment of TED regardless of disease activity or duration1
TED is a debilitating and potentially disfiguring disease3,12-14
AUTOIMMUNE DISORDER
Insulin-like growth factor-1 receptor (IGF-1R) drives pathophysiology throughout the disease course3,12,13,15
LIFELONG & PROGRESSIVE
Can potentially threaten vision and reactivate or flare over time3,12,13,16,17
HIGHLY VARIABLE
Signs and symptoms differ from patient to patient3,18
SYMPTOMS
Different diseases can sometimes be misdiagnosed due to similar symptoms12,13,19
ATA, American Thyroid Association; ETA, European Thyroid Association; TSH, thyroid-stimulating hormone.
DRY EYE DISEASE: Affects lacrimal glands and ocular surface causing insufficient lubrication to aqueous tear-film layer
TED and Graves’ disease have different underlying mechanisms and require different treatments3,12,13,21
Diagnose early and treat TED proactively
Early intervention can help improve patient outcomes and avoid potentially permanent damage13,22
Evaluate patients for visible signs and symptoms3,22
Ask patients about non-visible signs and symptoms3,22
Not all signs of TED may be visible, so it’s important to ask your patients if they’re experiencing new or changing symptoms. Consider a TED treatment that works at a root cause of the disease.1,3,22
Risk factors can include: smoking (past or present), radioactive iodine treatment, advanced age, female sex, poorly controlled hyper-/ hypothyroidism, and family history of TED or autoimmune diseases22,23
Hear how Dr Lisa Mihora uses a team approach to treat her patients with TED, and learn about the importance of early diagnosis
-
Diagnosis and treatment of TED
-
Read transcript
Treating TED is a team effort
Although TED and Graves’ disease may occur simultaneously, they are distinct entities and are caused by different underlying mechanisms.3,12,13,21 The understanding of TED has changed since insulin-like growth factor-1 receptor (IGF-1R) has been identified.3,12,13 Upregulation of orbital fibroblasts is a key driver of the pathophysiology of the disease and can cause inflammation and over-expansion of tissue leading to different clinical manifestations.15 Patients benefit from early treatment using a team approach between endocrinology, and ophthalmology or oculoplastics.20
Hi. My name is Dr Lisa Mihora. And I am an oculoplastics surgeon in Phoenix, Arizona. Distinguishing Thyroid Eye Disease and Graves’ disease is important. They’re often linked, but they are two different entities. And Thyroid Eye Disease can actually present before, during, or after the diagnosis of Graves’ disease.
Thyroid Eye Disease can present in patients who are euthyroid, hyperthyroid, or even hypothyroid. And because Thyroid Eye Disease is a separate and distinct disease from Graves’, treating Graves’ does not address the pathophysiology or symptomatology of Thyroid Eye Disease.
In Graves’ disease autoantibodies target the thyrotropin receptor, which thereby triggers hyperthyroidism. Whereas in Thyroid Eye Disease, we have additional autoantigens and antibodies that are involved.
The understanding of the mechanism of Thyroid Eye Disease has changed, now that insulin-like growth factor-1 receptor, or IGF-1 receptor, has been identified.
We now know that orbital fibroblasts, which are up-regulated in Thyroid Eye Disease, are key drivers of the pathophysiology of Thyroid Eye Disease. The T-cells and fibroblasts activate, and the inflammatory response and cascade has begun.
And the pathophysiology can translate into the signs and symptoms of Thyroid Eye Disease.
Once the fibroblasts are activated, they can cause severe inflammation and over-expansion of tissues, muscle, and the fat cells that are located behind the eye.
Because this is a fixed bony orbit, this can lead to different clinical manifestations.
Inflammation can occur, as well as foreign body sensation. A patient may have excessive tearing or dry eye. There can be conjunctival or eyelid redness, as well as swelling. A patient may have orbital pain, chemosis, proptosis, or bulging eye, and diplopia, or double vision.
Because there are so many different signs and symptoms that a patient can present with, educating patients, as well as our providers, means that we can hone in on the diagnosis earlier, and potentially treat earlier.
The goal of treating Thyroid Eye Disease early, in order to help combat the symptoms a patient may have, is a team approach. A team approach between endocrinology, and ophthalmology or oculoplastics.
Endocrinology has a very unique role, in that they specialise in treating the autoimmune disorder and the endocrine dysfunctions, such as Graves’ disease and refer early to the ophthalmologist or oculoplastic surgeon, in order to monitor the eye symptomatology.
Ophthalmologists or oculoplastic surgeons can often be the first to diagnose Thyroid Eye Disease patients. A baseline eye exam is conducted. And the patient’s Thyroid Eye Disease is evaluated.
I do co-manage patients with endocrinologists. And I find it very helpful when the endocrinologist now refers a patient early. That way, we can potentially start treatment, and start exams, and start the discussion, as early as possible.
I think that this dual approach to a patient gives a patient the best information and the best team approach, so that both aspects of the Thyroid Eye Disease can be effectively treated and managed.
Burden of TED
TED can have a debilitating impact on daily activities3,12-14
Vision may also be threatened24,25
62% of patients
experienced Proptosis26*
51% of patients
experienced diplopia27†
6% to 9% of patients
experienced dysthyroid optic neuropathy25,28,29‡
* Based on an incidence cohort of 120 patients with Graves’ orbitopathy in Olmsted County, Minnesota, who were diagnosed between 1976 and 1990.26
† Based on a cross-sectional follow-up study carried out from 1998 to 2000 of 168 patients with Graves’ orbitopathy who had started radiotherapy and/or prednisone treatment between 1982 and 1992.27
‡Based on a retrospective study of 1463 cases seen at the University of British Columbia Orbital Clinic between September 1976 and March 1986.29
TED may impact daily activities and emotional well-being14,30
66% of patients
suffered a high impact on daily life14§
42% of patients
experienced anxiety and/or depression30||
36% of patients
reported mental health issues14§
§ Based on retrospective chart review of 714 US patients by assessing physician-perceived impact of moderate-to-severe TED on patients’ quality of life (QOL). High overall QOL impact–score ≥4/7, where 1 meant “not at all impaired” and 7 meant “extremely impaired.14
‖ Based on an online QOL assessment survey of 100 patients including all levels of TED severity.30
Ask your patients about how the burden of TED effects them, including signs and symptoms, emotional well-being, and daily activity performance
DIAGNOSIS
Diagnosing TED
There are a number of diagnostic protocols and tools available to aid in a TED diagnosis. Radiographic imaging with CT or MRI can be helpful. Grading systems include the EUGOGO, VISA, and GO-QOL scales. The Clinical Activity Score (CAS) may be used to identify the signs and symptoms of inflammation characteristic of TED18,22,25
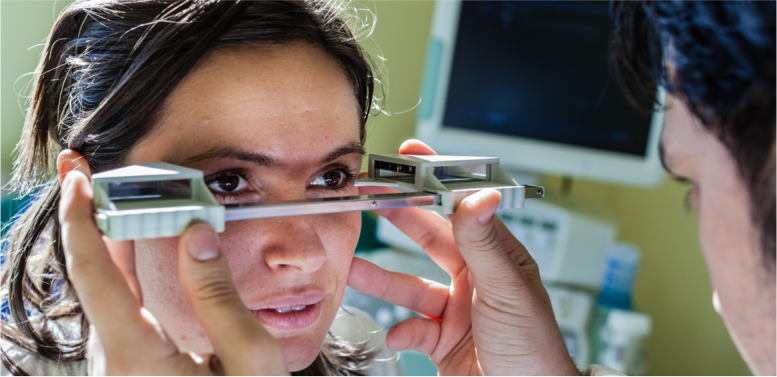
CAS measurements may be required for treatment approval
- The score at each efficacy assessment is the number of all items present31
Clinical Activity Score
- CAS is a 7-point composite score measuring spontaneous orbital pain, gaze-evoked orbital pain, eyelid swelling, eyelid erythema, conjunctival redness, chemosis, and inflammation of caruncle or plica. A lower score indicates fewer symptoms but does not take symptom severity into account. The CAS is a composite score with equal weighting of a number of factors. However, the factors may not be of equal clinical weight to patients or to physicians treating these patients31
ABOUT TEPEZZA
TEPEZZA prevents disease progression by treating a root cause of TED1,2,9,12,32
TEPEZZA is designed to target and block IGF-1R, a key driver of TED pathophysiology throughout the course of the disease.1,3,9,12,32 By inhibiting IGF-1R, TEPEZZA prevents muscle and tissue expansion behind the eye to help reduce TED symptoms.3,9,32
See TEPEZZA in action
-
Mechanism of action
-
Read transcript
TEPEZZA can reduce symptoms of TED by blocking IGF-1R
TED is a potentially vision-threatening autoimmune disease. It is caused by upregulation of orbital fibroblasts via autoantibodies triggering the IGF-1R/TSHR signalling complex. The activation of fibroblasts can cause severe inflammation and over-expansion of fat and muscle tissue behind the eye. This may lead to downstream effects like proptosis, diplopia, and eyelid retraction.3,12 TEPEZZA is the first and only approved treatment for TED.1,2 Through its novel, breakthrough mechanism TEPEZZA targets and blocks IGF-1R, thus, inhibiting fibroblast activation by the IGF-1R/TSHR signalling complex.1,3
Welcome, doctor.
You are here to learn how to identify and treat a rare and potentially debilitating disease affecting the human eye.
Although sometimes referred to as Graves’ orbitopathy, Thyroid Eye Disease, or T-E-D, is separate and distinct from Graves’ disease and has a unique underlying mechanism.Treating Graves’ may not lead to improvement in the clinical manifestations of T-E-D.
In Graves’ disease, autoantibodies target the thyroid-stimulating hormone receptor, triggering hyperthyroidism.
T-E-D is a potentially vision-threatening, chronic autoimmune disease with a heterogeneous presentation.
The reason for this difference lies deep within the human body.
Prepare to take a closer look.
In T-E-D, IGF-1R, a key mediator in the disease, and TSHR are colocalised and physically linked to form a functional signalling complex.
Central to the pathogenesis, autoantibodies activate the IGF-1R/TSHR signalling complex, which stimulates orbital fibroblasts, which can remain metabolically active throughout the course of the disease.
Once activated, these fibroblasts can cause potentially severe inflammation and expansion of muscle and fat behind the eye, leading to potentially debilitating downstream effects that are typical of T-E-D, such as proptosis, diplopia, and eyelid retraction.
Common inflammatory effects include, eyelid swelling and erythema, conjunctival redness, chemosis, and inflammation of the caruncle and plica.
Fortunately, you have a treatment option available with TEPEZZA, the first and only SFDA-approved treatment for T-E-D.
Through its novel, breakthrough mechanism, TEPEZZA targets and blocks IGF-1R and inhibits fibroblast activation via the IGF-1R/TSHR signalling complex.
IGF-1R inhibition on the surface of the orbital fibroblast is thought to attenuate downstream molecular events such as hyaluronan production, adipogenesis, muscle expansion, and inflammatory cytokine production.
By blocking IGF-1R, treatment with TEPEZZA has been proven to improve proptosis, diplopia, and inflammatory effects in T-E-D patients.
Congratulations, doctor, you now have the information to accurately identify T-E-D and understand the role of TEPEZZA in treating Thyroid Eye Disease.
IGF-1R, insulin-like growth factor-1 receptor; SFDA, Saudi Food and Drug Authority; TSHR, thyroid-stimulating hormone receptor.
Phase 2/3 Clinical Trial Efficacy
Rapid and clinically meaningful reductions in proptosis1,9
Phase 3 study:
Patients achieving ≥2-mm reduction in proptosis* at Week 24 (primary endpoint)9
Proptosis reduction as early as Week 6 occured in 56 % of TEPEZZA patients vs 7 % of placebo patients (P<0.01)
*A proptosis responder was defined as having a ≥2-mm reduction in proptosis from baseline in the study eye without deterioration (≥2-mm increase in proptosis) in the nonstudy eye.9
Rapid and continuous reductions in proptosis were significant1,9
Phase 3 study:
Mean change from baseline in proptosis (mm) over 24 weeks (secondary endpoint)9
All TEPEZZA patients completing treatment had proptosis reduction–it is important that patients complete the full treatment course of 8 IV infusions, as studied in clinical trials9
Durable proptosis improvement maintained nearly 1 year after eighth infusion—53% of patients (16 of 30) with proptosis reduced by ≥2 mm at Week 24 maintained proptosis reduction through Week 72 (Phase 2 study)1
IV, intravenous.
Complete resolution of diplopia in twice as many patients vs placebo1,9,10
Phase 2 and 3 studies:
Diplopia resolution‡ rate (grade 0) at Week 241
Durable diplopia improvement‡ maintained nearly 1 year after eighth infusion—67% of patients (12 of 18) with diplopia improved by ≥1 grade at Week 24 maintained diplopia improvement through Week 72 (Phase 2 study)1
‡Diplopia was evaluated on a 4-point scale ranging from 0 for no diplopia to 3 for constant diplopia. Diplopia resolution was defined as a patient with baseline diplopia >0 and a score of 0 at Week 24.1
-
Bahn-Gorman Scale
Bahn-Gorman scale is an ophthalmology tool designed to measure diplopia31
A change ≥1 grade is considered clinically meaningful.31
Diplopia Score
Diplopia was evaluated on a 4-point scale ranging from 0 for no diplopia to 3 for constant diplopia, the most severe grade.31
0: No diplopia
Diplopia absent1: Intermittent
Diplopia in primary position of gaze, when tired or first awakening2: Inconstant
Diplopia at extremes of gaze3: Constant
Continuous diplopia in primary or reading position
Pain, redness, and swelling improved during treatment34
Phase 2 and 3 studies:
Mean change from baseline in CAS over 24 weeks34
TEPEZZA is proven to treat more than just proptosis—more patients (TEPEZZA vs placebo) had complete resolution across ALL CAS symptoms34
CAS, Clinical Activity Score.
Inflammatory symptoms assessed with 7-point CAS included34
Spontaneous orbital pain
Gaze-evoked orbital pain
Conjunctival redness
Chemosis
Eyelid swelling
Eyelid erythema
Inflammation of caruncle or plica
Improved functional vision and patient appearance10||
Functional Vision
TEPEZZA improved functional vision, as defined by a patient’s ability to perform daily activities(eg, read, watch TV)10
Patient Appearance
TEPEZZA improved patient appearance, so patients no longer need to hide behind sunglasses or have the perception of being watched10
|| Patient-reported based on GO-QOL scale. GO-QOL, Graves' ophthalmopathy quality of life.
-
TEPEZZA inclusion/exclusion criteria and study design
Patients with high disease activity and short duration TED studied in two 24-week, randomised, double-masked, placebo-controlled trials1,9,10
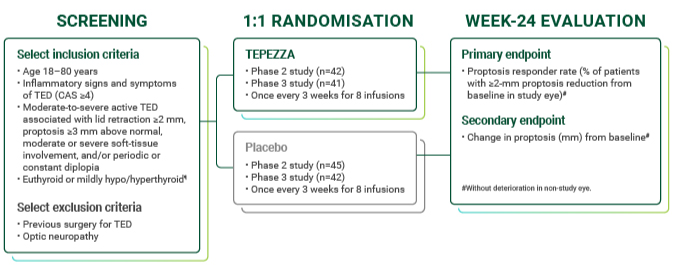
Largest clinical trial program for TED1,36
~ 8 out of 10 TED Patients
would prefer IV medicine before trying eye surgery37§
§ From a patient survey of 102 TED patients. Results based on a 5-point agreement scale; 79.4% completely/somewhat agreed with the statement “I would prefer prescription intravenous (IV) medicine before trying eye surgery.”37
Hear how TEPEZZA has changed the treatment landscape in TED
-
Clinical practice
-
Read transcript
TEPEZZA can improve patients' quality of life
TED may present itself in a variety of manifestations. These can include proptosis, pain and swelling around the eyes as well as double vision. Symptoms like double vision can severely impact a patient’s everyday life. Routine activities like grocery shopping, driving, or reading may become a challenge.8,12,30 In patients with TED, TEPEZZA has been shown to improve proptosis, double vision, the pain and redness associated with the disease, and, furthermore, their functional vision, and appearance.1,9,10
I’m Raymond Douglas and I’m an oculoplastic and orbital surgeon here in Los Angeles. Since the approval of TEPEZZA, it has really become first line in my treatment algorithm for patients.
Patients present with Thyroid Eye Disease with a very heterogenous group of symptoms and signs as we call them. And so they may present with just proptosis or double vision or eye swelling, et cetera. Patients with double vision have a significantly harder time just dealing with going to a grocery store or being able to drive. It affects their daily life. A particular patient comes to mind: she had swelling around the eyes that was red, often painful. Deep pain around the eyes, which can be very common, and an inability to look up. So any time she tried to read, any time she tried to look at the computer, one time it’s in focus, and the next time she’s seeing double. After receiving the full course of TEPEZZA treatment, the eye that was normal stayed pretty much normal and the same. But the eye that was affected by Thyroid Eye Disease that was bulging and couldn’t look up had now come back about three millimeters in proptosis. And now she had nearly full range of movement. So that now she could look down and look up without seeing double.
So when patients start to see the results of TEPEZZA, usually it’s relatively early in their treatment. And usually it’s accompanied by a “hug” moment. And they’re not fully there yet. And we still have to finish the course of therapy, but for the first time now they’re seeing an improvement that they haven’t seen.
TEPEZZA in patients with Thyroid Eye Disease has been shown to improve the proptosis, double vision, the pain and redness associated with Thyroid Eye Disease, their functional vision, and their appearance.
For me, it’s an honour to participate in their care and to be able to offer a treatment that hopefully will help them throughout this process.
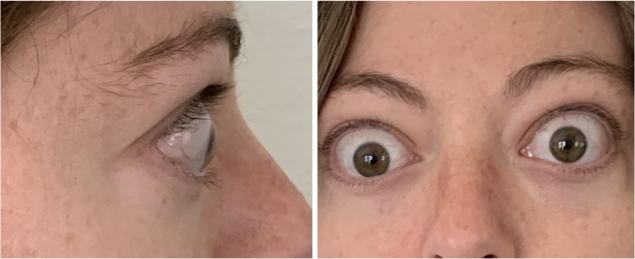
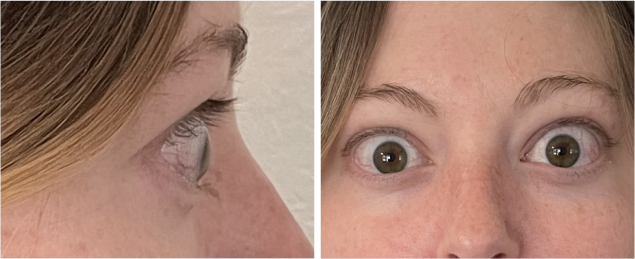
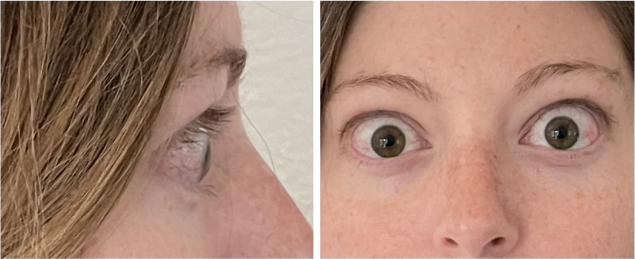
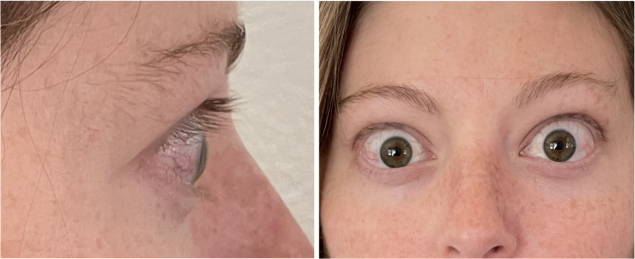
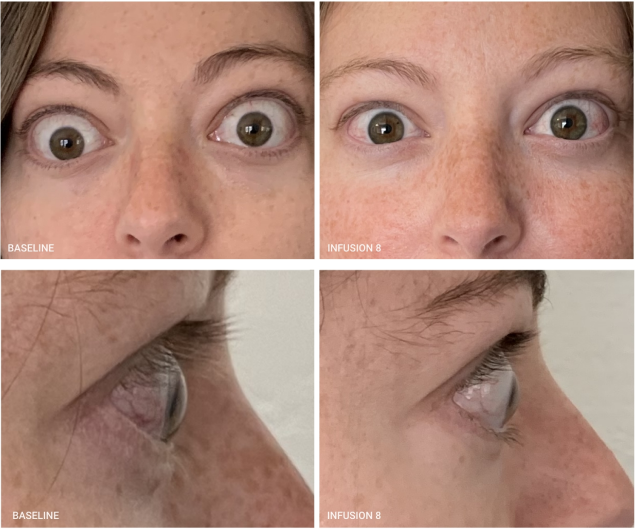
Real Patient Results
TEPEZZA has been shown to be effective in TED patients with a wide range of clinical manifestations6,7,9
See results for patients treated with TEPEZZA in Phase 2/3 clinical trials
Patient treated with TEPEZZA in a clinical trial. Patient completed a full course (8 IV infusions) of treatment with TEPEZZA. Results shown are with no surgical intervention. Individual results may vary.9
IV, intravenous.
See more results for additional patients treated with TEPEZZA throughout the full course of 8 IV infusions
Case of patient with TED and their individual experience with TEPEZZA. Individual results may vary. Clinical presentations represent a range of patients with TED. Patients with prior surgery or orbital radiation treatment for TED, history of compressive optic neuropathy, and concomitant steroid use were excluded from clinical trials for TEPEZZA.
OD, oculus dexter (right eye); OS, oculus sinister (left eye); OU, oculus uterque (both eyes).
ADDITIONAL STUDY DATA
Decreased proptosis in patients who had a disease flare and were retreated40
OPTIC-X study:
Proptosis response over 24 weeks of retreatment with TEPEZZA40
For patients who had a proptosis response at Week 24 and then experienced a flare,* the majority (63%; 5 of 8) achieved improvements in proptosis (≥2-mm reduction from OPTIC-X baseline) with a second course of TEPEZZA at Week 24 in OPTIC-X40†
The OPTIC-X patients who did not experience a ≥2-mm improvement in proptosis (n=3) had reductions relative to their OPTIC baseline (3 mm, 3 mm, and 4 mm, respectively)40
* Defined by general presence of symptoms indicating flare, in addition to at least 1 of the following: increase in proptosis of ≥2 mm in the study eye since Week 24 or an absolute CAS of at least 4 in the study eye with a ≥2-point increase.40
†Only 8 patients contributed to data at Week 24, as 1 patient had a significantly delayed visit due to COVID-19 and was excluded from Week 24 analysis per the statistical analysis plan.40
-
OPTIC-X study design
OPTIC-X evaluated safety and efficacy of TEPEZZA in patients who were proptosis nonresponders at OPTIC Week 24 or proptosis responders at Week 24 but flared during 48-Week follow-up period.40

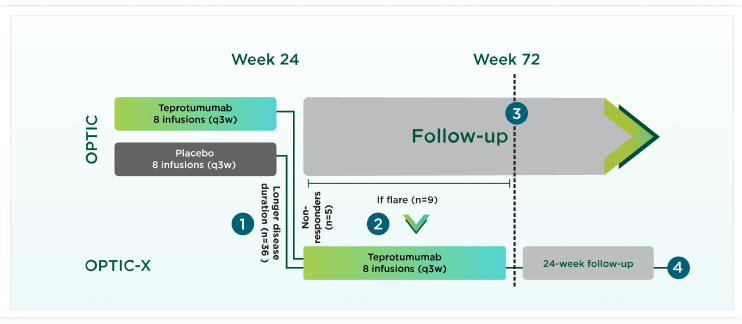
A flare was defined as
Patients losing ≥2 mm of their Week 24 proptosis improvement during the 48-week OPTIC follow-up period, even if their proptosis was still substantially better than at OPTIC baseline40
-OR-
Patients experiencing an increase in CAS of at least 2 points from OPTIC Week 24, with an absolute CAS of at least 4 in the study eye40
-AND-
Presence of symptoms40
TEPEZZA Safety
Proven efficacy with a demonstrated safety and tolerability profile1,9,10
Low discontinuation rate1
Most adverse events were mild or moderate, manageable, and resolved during or after treatment9,10
Adverse reactions occurring in ≥5% of patients treated with TEPEZZA with greater incidence than placebo1
| Adverse reactions | TEPEZZA N=84, n (%) | Placebo N=86, n (%) |
|---|---|---|
| Muscle spasms | 21 (25%) | 6 (7%) |
| Nausea | 14 (17%) | 8 (9%) |
| Alopecia | 11 (13%) | 7 (8%) |
| Diarrhoea | 10 (12%) | 7 (8%) |
| Fatiguea | 10 (12%) | 6 (7%) |
| Hyperglycaemiab | 8 (10%) | 1 (1%) |
| Hearing impairmentc | 8 (10%) | 0 |
| Dysgeusia (taste disturbance) | 7 (8%) | 0 |
| Headache | 7 (8%) | 6 (7%) |
| Dry skin | 7 (8%) | 0 |
aFatigue includes asthenia.1
bHyperglycaemia includes blood glucose increase.1
cHearing impairment including hearing loss (deafness, including sensorineural deafness, eustachian tube dysfunction, hyperacusis, hypoacusis, autohpony and tinnitus).1
Prescribing TEPEZZA
TEPEZZA IS A PRESCRIPTION MEDICATION ADMINISTERED VIA IV INFUSION
A full course of treatment with TEPEZZA is 8 IV infusions, given in the arm once every 3 weeks.1 TEPEZZA may be administered at an infusion center, your practice, a hospital, or your patient’s home.
Dosing information
Patients should complete the full treatment course to get the full benefits1
- TEPEZZA dosing is based on the patient’s actual weight1
- The initial dose is 10 mg/kg. The dose for Infusions 2 to 8 is 20 mg/kg1
It is important that patients complete the full TEPEZZA treatment course of 8 IV infusions as studied in clinical trials.1
Dosing Calculations
Determine the dosing and infusion values for your patient1
Patients weighing 50 kg to 85 kg
| Patient Weight | Infusion 1 (10 mg/kg) | Infusions 2 to 8 (20 mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| lb* | kg† | Dose (mg) | Vials required (#) | Volume to withdraw (mL)† | Dose (mg) | Vials required (#) | Volume to withdraw (mL)† |
| 110 | 50 | 500 | 1 | 10.5 | 1000 | 2 | 21 |
| 112 | 51 | 510 | 2 | 10.7 | 1020 | 3 | 21.4 |
| 115 | 52 | 520 | 2 | 10.9 | 1040 | 3 | 21.8 |
| 117 | 53 | 530 | 2 | 11.1 | 1060 | 3 | 22.3 |
| 119 | 54 | 540 | 2 | 11.3 | 1080 | 3 | 22.7 |
| 121 | 55 | 550 | 2 | 11.6 | 1100 | 3 | 23.1 |
| 123 | 56 | 560 | 2 | 11.8 | 1120 | 3 | 23.5 |
| 126 | 57 | 570 | 2 | 12 | 1140 | 3 | 23.9 |
| 128 | 58 | 580 | 2 | 12.2 | 1160 | 3 | 24.4 |
| 130 | 59 | 590 | 2 | 12.4 | 1180 | 3 | 24.8 |
| 132 | 60 | 600 | 2 | 12.6 | 1200 | 3 | 25.2 |
| 134 | 61 | 610 | 2 | 12.8 | 1220 | 3 | 25.6 |
| 137 | 62 | 620 | 2 | 13 | 1240 | 3 | 26.1 |
| 139 | 63 | 630 | 2 | 13.2 | 1260 | 3 | 26.5 |
| 141 | 64 | 640 | 2 | 13.4 | 1280 | 3 | 26.9 |
| 143 | 65 | 650 | 2 | 13.7 | 1300 | 3 | 27.3 |
| 146 | 66 | 660 | 2 | 13.9 | 1320 | 3 | 27.7 |
| 148 | 67 | 670 | 2 | 14.1 | 1340 | 3 | 28.2 |
| 150 | 68 | 680 | 2 | 14.3 | 1360 | 3 | 28.6 |
| 152 | 69 | 690 | 2 | 14.5 | 1380 | 3 | 29 |
| 154 | 70 | 700 | 2 | 14.7 | 1400 | 3 | 29.4 |
| 157 | 71 | 710 | 2 | 14.9 | 1420 | 3 | 29.8 |
| 159 | 72 | 720 | 2 | 15.1 | 1440 | 3 | 30.3 |
| 161 | 73 | 730 | 2 | 15.3 | 1460 | 3 | 30.7 |
| 163 | 74 | 740 | 2 | 15.5 | 1480 | 3 | 31.1 |
| 165 | 75 | 750 | 2 | 15.8 | 1500 | 3 | 31.5 |
| 168 | 76 | 760 | 2 | 16 | 1520 | 4 | 31.9 |
| 170 | 77 | 770 | 2 | 16.2 | 1540 | 4 | 32.4 |
| 172 | 78 | 780 | 2 | 16.4 | 1560 | 4 | 32.8 |
| 174 | 79 | 790 | 2 | 16.6 | 1580 | 4 | 33.2 |
| 176 | 80 | 800 | 2 | 16.8 | 1600 | 4 | 33.6 |
| 179 | 81 | 810 | 2 | 17 | 1620 | 4 | 34 |
| 181 | 82 | 820 | 2 | 17.2 | 1640 | 4 | 34.5 |
| 183 | 83 | 830 | 2 | 17.4 | 1660 | 4 | 34.9 |
| 185 | 84 | 840 | 2 | 17.6 | 1680 | 4 | 35.3 |
| 187 | 85 | 850 | 2 | 17.9 | 1700 | 4 | 35.7 |
Patients weighing 86 kg to 120 kg
| Patient Weight | Infusion 1 (10 mg/kg) | Infusions 2 to 8 (20 mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| lb* | kg† | Dose (mg) | Vials required (#) | Volume to withdraw (mL)† | Dose (mg) | Vials required (#) | Volume to withdraw (mL)† |
| 190 | 86 | 860 | 2 | 18.1 | 1720 | 4 | 36.1 |
| 192 | 87 | 870 | 2 | 18.3 | 1740 | 4 | 36.6 |
| 194 | 88 | 880 | 2 | 18.5 | 1760 | 4 | 37 |
| 196 | 89 | 890 | 2 | 18.7 | 1780 | 4 | 37.4 |
| 198 | 90 | 900 | 2 | 18.9 | 1800 | 4 | 37.8 |
| 201 | 91 | 910 | 2 | 19.1 | 1820 | 4 | 38.2 |
| 203 | 92 | 920 | 2 | 19.3 | 1840 | 4 | 38.7 |
| 205 | 93 | 930 | 2 | 19.5 | 1860 | 4 | 39.1 |
| 207 | 94 | 940 | 2 | 19.7 | 1880 | 4 | 39.5 |
| 209 | 95 | 950 | 2 | 20 | 1900 | 4 | 39.9 |
| 212 | 96 | 960 | 2 | 20.2 | 1920 | 4 | 40.3 |
| 216 | 98 | 980 | 2 | 20.6 | 1960 | 4 | 41.2 |
| 218 | 99 | 990 | 2 | 20.8 | 1980 | 4 | 41.6 |
| 220 | 100 | 1000 | 2 | 21 | 2000 | 4 | 42 |
| 223 | 101 | 1010 | 3 | 21.2 | 2020 | 5 | 42.4 |
| 225 | 102 | 1020 | 3 | 21.4 | 2040 | 5 | 42.9 |
| 227 | 103 | 1030 | 3 | 21.6 | 2060 | 5 | 43.3 |
| 229 | 104 | 1040 | 3 | 21.8 | 2080 | 5 | 43.7 |
| 231 | 105 | 1050 | 3 | 22.1 | 2100 | 5 | 44.1 |
| 234 | 106 | 1060 | 3 | 22.3 | 2120 | 5 | 44.5 |
| 236 | 107 | 1070 | 3 | 22.5 | 2140 | 5 | 45 |
| 238 | 108 | 1080 | 3 | 22.7 | 2160 | 5 | 45.4 |
| 240 | 109 | 1090 | 3 | 22.9 | 2180 | 5 | 45.8 |
| 243 | 110 | 1100 | 3 | 23.1 | 2200 | 5 | 46.2 | 245 | 111 | 1110 | 3 | 23.3 | 2220 | 5 | 46.6 |
| 247 | 112 | 1120 | 3 | 23.5 | 2240 | 5 | 47.1 |
| 249 | 113 | 1130 | 3 | 23.7 | 2260 | 5 | 47.5 |
| 254 | 115 | 1150 | 3 | 24.2 | 2300 | 5 | 48.3 |
| 256 | 116 | 1160 | 3 | 24.4 | 2320 | 5 | 48.7 |
| 258 | 117 | 1170 | 3 | 24.6 | 2340 | 5 | 49.2 |
| 260 | 118 | 1180 | 3 | 24.8 | 2360 | 5 | 49.6 |
| 262 | 119 | 1190 | 3 | 25 | 2380 | 5 | 50 |
| 265 | 120 | 1200 | 3 | 25.2 | 2400 | 5 | 50.4 |
Saline bag size:
- If dose is <1800 mg, use a 100-mL bag of normal saline (0.9% NaCl). 1
- If dose is ≥1800 mg, use a 250-mL bag of normal saline (0.9% NaCl)1
IV, intravenous.
WARNINGS AND PRECAUTIONS
Important patient counselling information
Infusion reactions1
- Infusion reactions have been reported in approximately 4% of patients treated with TEPEZZA
- − Reported infusion reactions have usually been mild or moderate in severity
TEPEZZA may cause an exacerbation of preexisting inflammatory bowel disease (IBD)1
- Monitor patients for disease flare; if exacerbation is suspected, consider discontinuing TEPEZZA
Hyperglycaemia1
- In clinical trials, 10% of patients (two-thirds of whom had preexisting diabetes or impaired glucose tolerance) experienced hyperglycaemia
- Monitor patients for elevated blood glucose and symptoms of hyperglycaemia while on treatment with TEPEZZA
- Patients with preexisting diabetes or impaired glucose tolerance should be under appropriate glycaemic control before receiving TEPEZZA
Hearing impairment including hearing loss
- TEPEZZA may cause severe hearing impairment including hearing loss, which in some cases may be permanent1,9,10
- Assess patients’ hearing before, during, and after treatment with TEPEZZA and consider the benefit-risk of treatment with patients
- Instruct patients to contact their healthcare provider if they experience any signs or symptoms of hearing impairment or any changes in hearing
Pregnancy1
- Women of childbearing potential need to use effective contraception prior to initiation, during treatment with TEPEZZA, and for 6 months after the last dose
-
REFERENCES
- TEPEZZA prescribing information.
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-thyroid-eye-disease (Accessed May 2024).
- Patel A, Yang H, Douglas RS. A new era in the treatment of thyroid eye disease. Am J Ophthalmol. 2019;208:281-288.
- Chen H, Mester T, Raychaudhuri N, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99(9):E1635-E1640.
- Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397-4405.
- Diniz SB, Cohen LM, Roelofs KA, et al. Early experience with the clinical use of teprotumumab in a heterogenous thyroid eye disease population. Ophthalmic Plast Reconstr Surg. 2021;37(6):583-591.
- Ugradar S, Kang J, Kossler AL, et al. Teprotumumab for the treatment of chronic thyroid eye disease. Eye (Lond). 2021;36(8):1553-1559.
- Bruscolini A, Sacchetti M, La Cava M, et al. Quality of life and neuropsychiatric disorders in patients with Graves’ orbitopathy: current concepts. Autoimmun Rev. 2018;17(7):639-643.
- Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020;382(4):341-352.
- Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N Engl J Med. 2017;376(18):1748-1761.
- Ponto KA, Merkesdal S, Hommel G, et al. Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98(1):145-152.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726-738.
- Wang Y, Patel A, Douglas RS. Thyroid Eye Disease: how a novel therapy may change the treatment paradigm. Ther Clin Risk Manag. 2019;15:1305-1318.
- Wang Y, Sharma A, Padnick-Silver L, et al. Physician-perceived impact of Thyroid Eye Disease on patient quality of life in the United States. Ophthalmol Ther. 2021;10(1):75-87.
- Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res. 2016;142:83-91.
- Patel P, Khandji J, Kazim M. Recurrent Thyroid Eye Disease. Ophthal Plast Reconstr Surg. 2015;31(6):445-448.
- Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety and durability in longer-duration Thyroid Eye Disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129(4):438-449.
- Bothun ED, Scheurer RA, Harrison AR, Lee MS. Update on Thyroid Eye Disease and management. Clin Ophthalmol. 2009;3:543-551.
- Verjee MA, Brissette AR, Starr CE. Dry eye disease: early recognition with guidance on management and treatment for primary care family physicians. Ophthalmol Ther. 2020;9:877-888.
- Burch HB, Perros P, Bednarczuk T, et al. Management of Thyroid Eye Disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022;32(12):1439-1470.
- Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552-1665.
- Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC. Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J Ophthalmol. 2015;2015:249125.
- Risk factors for the development of Thyroid Eye Disease in patients with Graves’ disease. Clin Thyroidology for the Public. 2021;14(8):5-6.
- Ponto KA, Merkesdal S, Hommel G, Pitz S, Pfeiffer N, Kahaly GJ. Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98(1):145-152.
- McAlinden C. An overview of Thyroid Eye Disease. Eye Vis (Lond). 2014;1:9.
- Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121(3):284-290.
- Terwee C, Wakelkamp I, Tan S, Dekker F, Prummel MF, Wiersinga W. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146(6):751-757.
- Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92(1):477-588.
- Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmol. 1988;95(11):1515-1521.
- Cockerham KP, Padnick-Silver L, Stuertz N, Francis-Sedlak M, Holt RJ. Quality of life in patients with chronic Thyroid Eye Disease in the United States. Ophthalmol Ther. 2021;10(4):975-987.
- Wiersinga WM, Perros P, Kahaly GJ, et al. Clinical assessment of patients with Graves’ orbitopathy: the European Group on Graves’ Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol. 2006;155(3):387-389.
- Douglas RS. Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active Thyroid Eye Disease: a focus on proptosis. Eye (Lond). 2019;33(2):183-190.
- Data on File. Horizon, November 2020.
- Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active Thyroid Eye Disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled multicentre trials. Lancet. 2021;9(6):360-372.
- Data on File. Horizon, May 2022.
- Rollet J. Symptoms, quality of life improve with teprotumumab for adults with Thyroid Eye Disease. Endocrine Today. October 31, 2019. https://www.healio.com/news/endocrinology/20191031/symptoms-quality-of-life-improve-with-teprotumumab-for-adults-with-thyroid-eye-disease (Accessed May 2024).
- Data on File. Horizon, April 2023.
- Data on File. Horizon, January 2020.
- Ozzello DJ, Dallalzadeh LO, Liu CY. Teprotumumab for chronic Thyroid Eye Disease. Orbit. 2022;41(5):539-546.
- Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety and durability in longer duration Thyroid Eye Disease and retreatment: OPTIC-X study. Ophthalmol. 2022:129(4):438-449.
-
ABBREVIATED PRODUCT INFORMATION
Tepezza (teprotumumab)
Brief Prescribing Information: Please refer to the Summary of Product Characteristics before prescribing Tepezza. Pharmaceutical Form: Lyophilisate for solution for infusion. White to off-white, lyophilised powder for intravenous infusion. Upon reconstitution, Tepezza is a colourless or slightly brown, clear to opalescent solution which is free of foreign particulate matter. The solution has a pH of approximately 5.5. Therapeutic indications: Tepezza is indicated for the treatment of thyroid eye disease. Posology and method of administration: Administration precautions: Treatment should be initiated under the supervision of a physician experienced in the treatment of thyroid eye disease and with access to appropriate medical support to manage potential severe reactions such as serious infusion-related reactions. Posology: The recommended dose of Tepezza is an intravenous infusion of 10 mg/kg for the initial dose followed by an intravenous infusion of 20 mg/kg every 3 weeks for 7 additional infusions. Special populations: Paediatric population. The safety and efficacy of teprotumumab in children and adolescents aged 0 to 18 years has not yet been established. No data are available. Method of administration Administer the diluted solution intravenously over 90 minutes for the first 2 infusions. If well tolerated, the minimum time for subsequent infusions can be reduced to 60 minutes. If not well tolerated, the minimum time for subsequent infusions should remain at 90 minutes. Do not administer as an intravenous push or bolus. Tepezza should not be infused concomitantly with other agents. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Special warnings and precautions for use: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Infusion-related reactions: Tepezza may cause infusion reactions. Infusion reactions have been reported in approximately 4% of patients treated with Tepezza. Signs and symptoms of infusion-related reactions include transient increases in blood pressure, feeling hot, tachycardia, dyspnoea, headache and muscular pain. Infusion reactions may occur during any of the infusions or within 1.5 hours after an infusion. Reported infusion reactions are usually mild or moderate in severity and can usually be successfully managed with corticosteroids and antihistamines. In patients who experience an infusion reaction, consideration should be given to pre-medicating with an antihistamine, antipyretic, corticosteroid and/or administering all subsequent infusions at a slower infusion rate. Exacerbation of pre-existing inflammatory bowel disease (IBD): Tepezza may cause an exacerbation of pre-existing IBD. Monitor patients with IBD for flare of disease. If IBD exacerbation is suspected, consider discontinuation of Tepezza. Hyperglycaemia: Hyperglycaemia or increased blood glucose may occur in patients treated with Tepezza. In clinical trials, 10% of patients (two thirds of whom had pre-existing diabetes or impaired glucose tolerance) experienced hyperglycaemia. Hyperglycaemic events should be controlled with medications for glycaemic control, if necessary. Monitor patients for elevated blood glucose and symptoms of hyperglycaemia while on treatment with Tepezza. Patients with pre-existing diabetes should be under appropriate glycaemic control before receiving Tepezza. Interaction with other medicinal products and other forms of interaction No interaction studies have been performed. Fertility, pregnancy, and lactation Women of childbearing potential: Based on its mechanism of action inhibiting insulin-like growth factor 1 receptor (IGF-1R), Tepezza may cause foetal harm when administered to a pregnant woman. Women of childbearing potential should use effective contraception (methods that result in less than 1% pregnancy rates) prior to initiation, during treatment with Tepezza and for 6 months after the last dose of Tepezza. Pregnancy: There are no or limited amount of data from the use of teprotumumab in pregnant women. Based on findings in animals and its mechanism of action inhibiting insulin-like growth factor 1 receptor (IGF-1R), Tepezza may cause foetal harm when administered to a pregnant woman. Adequate and well-controlled studies with Tepezza have not been conducted in pregnant women. There are insufficient data with Tepezza use in pregnant women to inform any drug associated risks for adverse developmental outcomes. In utero teprotumumab exposure in cynomolgus monkeys dosed once weekly with teprotumumab throughout pregnancy resulted in external and skeletal abnormalities. Teprotumumab exposure may lead to an increase in foetal loss. Therefore, Tepezza should not be used in pregnancy, and appropriate forms of contraception should be implemented prior to initiation, during treatment and for 6 months following the last dose of Tepezza. If the patient becomes pregnant during treatment, Tepezza should be discontinued, and the patient advised of the potential risk to the foetus. The background rate of major birth defects and miscarriage is unknown for the indicated population. In the general population, the estimated background risks of major birth defects and miscarriage in clinically recognised pregnancies are 2 – 4% and 15 – 20%, respectively. Based on mechanism of action inhibiting IGF-1R, postnatal exposure to teprotumumab may cause harm. Breast-feeding There is no information regarding the presence of Tepezza in human milk, the effects on the breast-fed infant or the effects on milk production. Fertility: Fertility studies have not been performed with Tepezza. Effects on ability to drive and use machines: The pharmacological activity and adverse reactions reported to date suggest that teprotumumab has no or negligible influence on the ability to drive and use machines. Undesirable effects: refer to SmPC for full information. Muscle spasms, Nausea, Alopecia, Diarrhoea, Fatigue, Hyperglycaemia, Hearing impairment, Dysgeusia, Headache and Dry skin. List of Adverse reactions: Hyperglycaemia, Hearing impairment, Nausea, Diarrhoea, Alopecia, Muscle spasms, Fatigue (very common), Dysgeusia, Headache, Dry skin, Infusion-related reaction (common), Exacerbation of IBD (Not known). Overdose No information is available for patients who have received an overdosage. Special precautions for storage: Store in refrigerator (2 °C – 8 °C) in original carton in order to protect from light. Do not freeze. For storage conditions after dilution of the medicinal product. Special precautions for disposal and other handling Reconstitution and preparation of infusion solution Step 1: Calculate the dose (mg) and determine the number of vials needed for the 10 or 20 mg/kg dosage based on patient weight. Each Tepezza vial contains 500 mg of the teprotumumab antibody. Step 2: Using appropriate aseptic technique, reconstitute each Tepezza vial with 10 mL of sterile water for injection. Ensure that the stream of diluent is not directed onto the lyophilised powder, which has a cake-like appearance. Do not shake, but gently swirl the solution by rotating the vial until the lyophilised powder is dissolved. The reconstituted solution has a volume of 10.5 mL. Withdraw 10.5 mL of reconstituted solution to obtain 500 mg. After reconstitution, the final concentration is 47.6 mg/mL. Step 3: The reconstituted Tepezza solution must be further diluted in 0.9% sodium chloride solution, prior to infusion. To maintain a constant volume in the infusion bag, a sterile syringe and needle should be used to remove the volume equivalent to the amount of the reconstituted Tepezza solution to be placed into the infusion bag. Discard the 0.9% sodium chloride, volume withdrawn. Step 4: Withdraw the required volume from the reconstituted Tepezza vial(s) based on the patient’s weight (in kg) and transfer into an intravenous bag containing 0.9% sodium chloride solution to prepare a diluted solution with a total volume of 100 mL (for less than 1,800 mg dose) or 250 mL (for 1,800 mg and greater dose). Mix diluted solution by gentle inversion. Do not shake. Discard the solution if any particulate matter or discoloration are observed. Disposal: Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Discard vial(s) and all unused contents. Legal category: POM. Marketing Authorisation Holder: Horizon Therapeutics Ireland DAC 70 St. Stephen’s Green Dublin 2 D02 E2X4 Ireland. Local Marketing authorization numbers: 2802245000. Date of revision of the text: Jan 2020
Local representative name and address; Cigalah Group of Companies. Address: Office Number 606&607, 6th floor, Al Akariya Building Number 2, Olaya Street, Riyadh, PIN 11533, Kingdom of Saudi Arabia.Tel: 00966 11 419 1471.
Any suspected adverse reactions should be reported immediately to Amgen in accordance with local spontaneous reporting requirements. Amgen Fax: +966 112 799328 or send to mailbox: Safety-MEA@amgen.com and/or National Pharmacovigilance Centre (NPC), Email: npc.drug@sfda.gov.sa, Fax: +966-11-2057662, SFDA Call Center 19999, website: http://ade.sfda.gov.sa